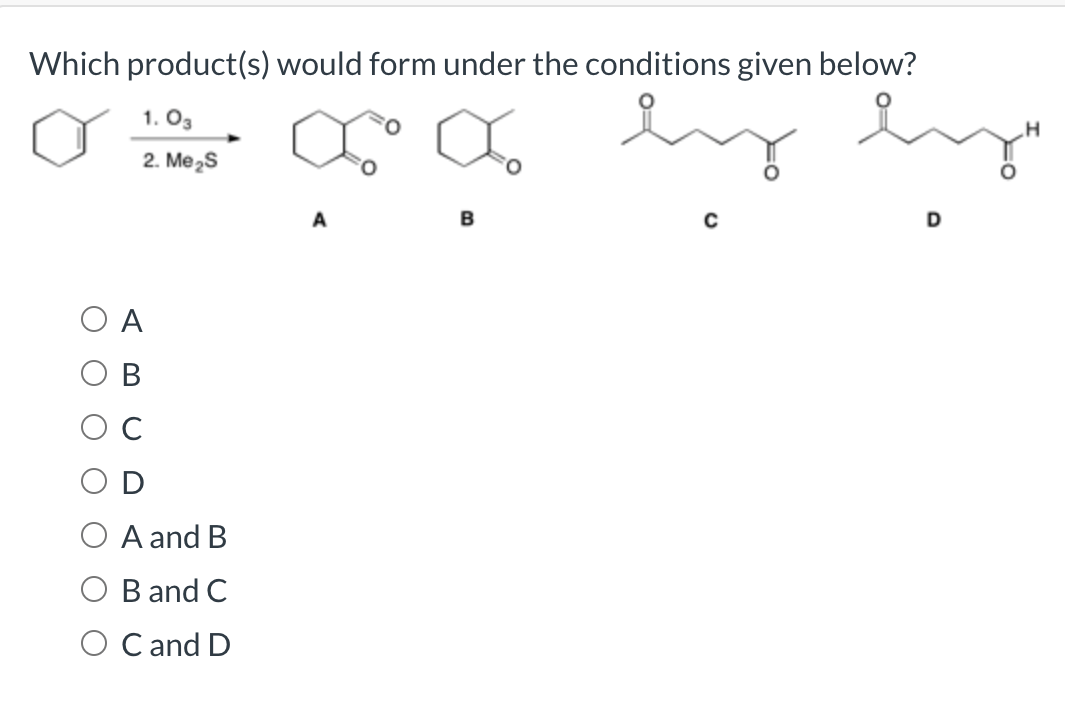
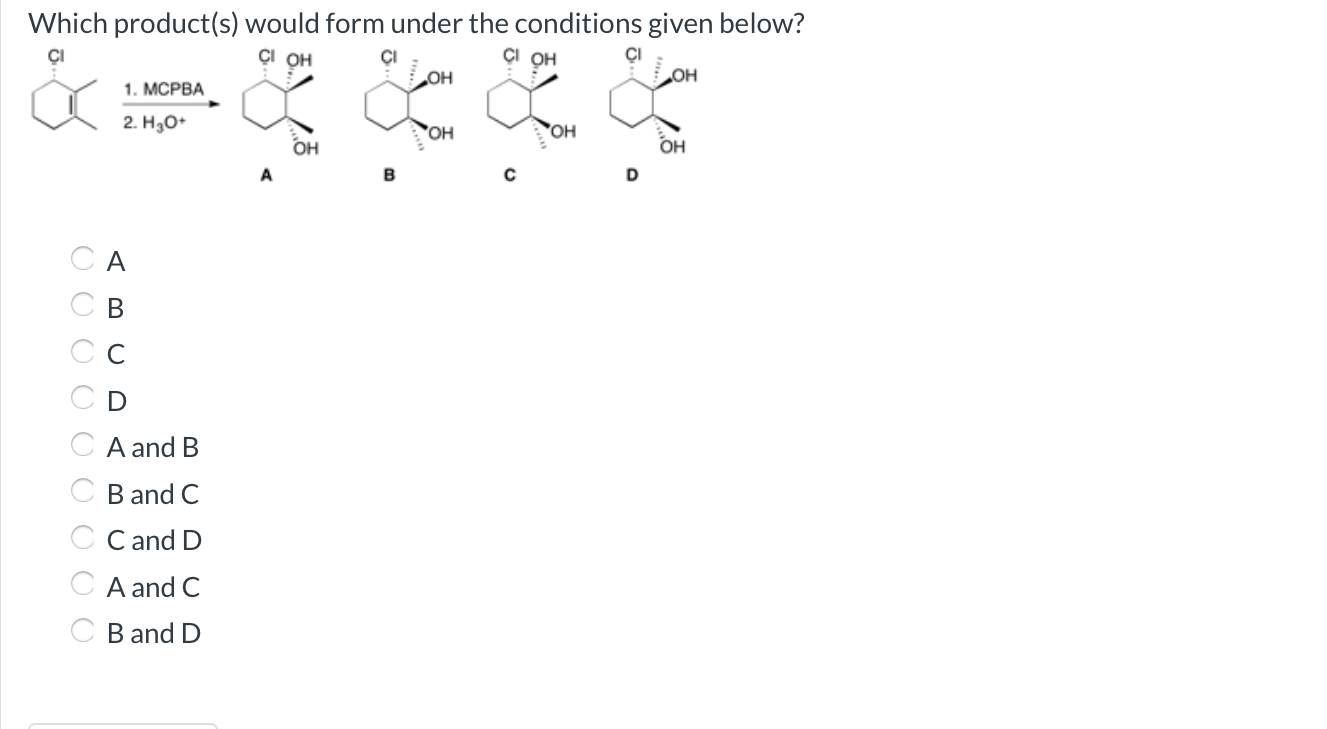
Which Product S Would Form Under The Conditions Given Below - Nmo oa ob oc od oa and b ob and c ob and d oc. A reaction with 4 possible bond line products is shown. Web the reaction can be represented as follows: A) 14.86 ml + 15.0 ml + 14.980 ml = b) (42.927 g/ml) (9.00 ml) = round off the answer. It is a dihydroxylation reagent, dry hydroxylation. Solution for which product (s) would form under the conditions given below? Oh + oh + h2so4 → ether + h2o therefore, the products. Web which product (s) would form under the conditions given below? Web which product (s) would form under the conditions given below? Me s a в с od н в.
So, according to this question, oso4 is osmium tetra oxide. Web which product (s) would form under the conditions given below? A reaction with 4 possible bond line products is shown. Solution for which product (s) would form under the conditions given below? Me s a в с od н в. Nmo oa ob oc od oa and b ob and c ob and d oc. Web which product (s) would form under the conditions given below? Oh + oh + h2so4 → ether + h2o therefore, the products. Web the reaction can be represented as follows: It is a dihydroxylation reagent, dry hydroxylation. A) 14.86 ml + 15.0 ml + 14.980 ml = b) (42.927 g/ml) (9.00 ml) = round off the answer.