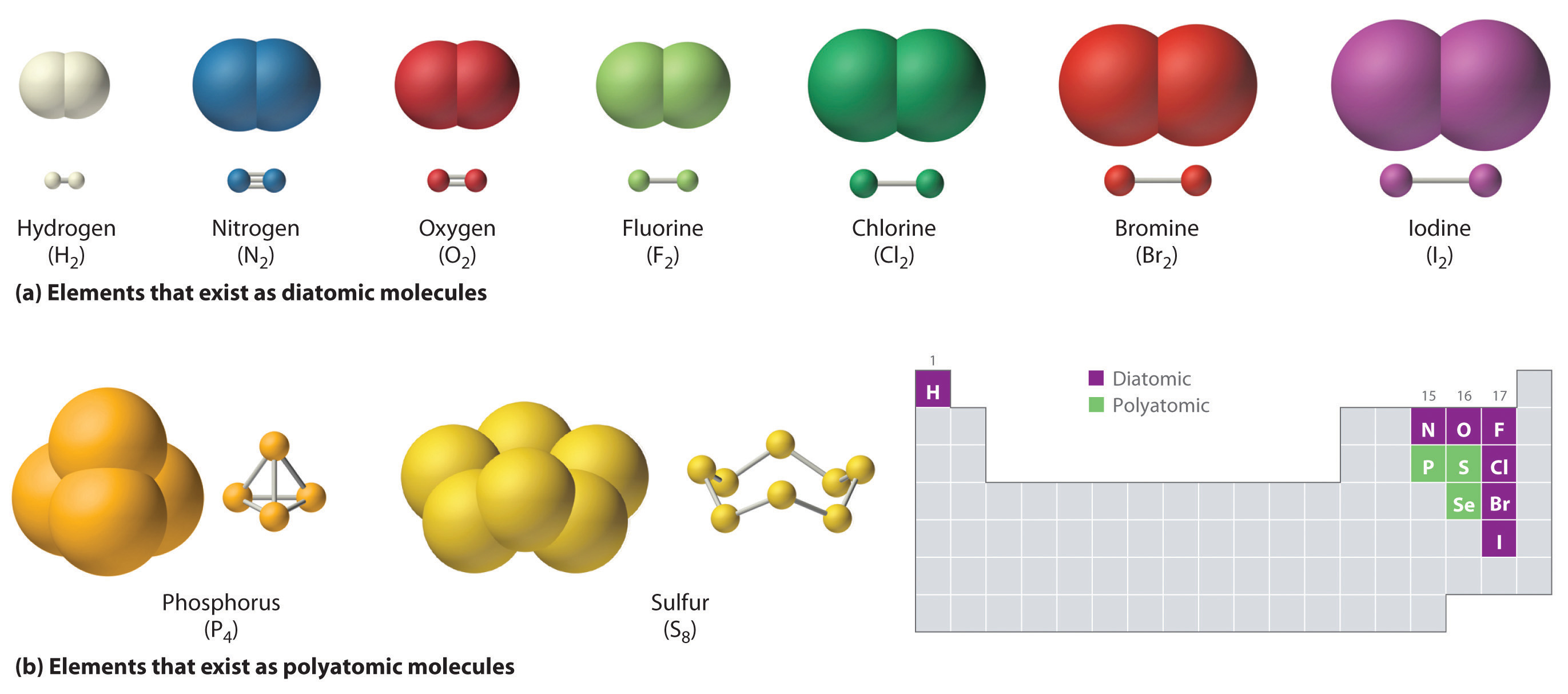
Which Pair Of Elements Will Form A Covalent Bond - Web covalent bonds involve shared electron pairs between atoms. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar. Web the most common examples are the covalent compounds of beryllium and boron. Each atom contributes one electron to each shared pair, and effectively gains an additional electron from the. For each molecule, there are different names for. Web the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. In pure covalent bonds, the electrons are shared. For example, beryllium can form two covalent bonds, resulting in only four. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms.
In pure covalent bonds, the electrons are shared. Web covalent bonds involve shared electron pairs between atoms. For example, beryllium can form two covalent bonds, resulting in only four. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar. Each atom contributes one electron to each shared pair, and effectively gains an additional electron from the. For each molecule, there are different names for. Web the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. Web the most common examples are the covalent compounds of beryllium and boron.